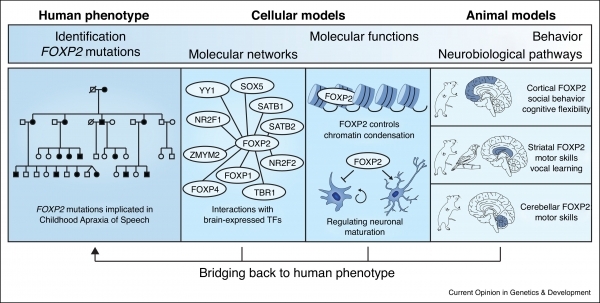
Following our discovery that rare FOXP2 disruptions cause a severe speech and language disorder, we generated models for mice carrying the same mutations as those found in the most well-studied human cases. (The mouse version of this gene, which is very similar to that in humans, is referred to as FOXP2.) These models have shed a light on the neurobiological pathways that go awry in the disorder. For example, our research revealed impairments in connectivity, plasticity, and excitatory/inhibitory balance in a subset of brain circuits, involving the cortex, basal ganglia and cerebellum, and demonstrated that the mice have impairments in the learning of motor skills and sequencing of ultrasonic vocalisations. We went on to develop models in which it is possible to selectively deactivate FOXP2 in different tissues and/or at different developmental time points. Such models enable us to map the relative contributions of this gene onto the functions of the cortex, basal ganglia, and cerebellum.
For this work, we collaborate closely with leading experts in basal ganglia and cerebellar functions as well as researchers experienced in the study of motor skills and vocal behaviours. In complementary research, we are involved in studies of genes related to FOXP2 in other animal models, including songbirds and fruit flies.
Example publications:
French, C. A., et al. (2019). Differential effects of Foxp2 disruption in distinct motor circuits. Molecular Psychiatry. 24(3), 447-462. doi:10.1038/s41380-018-0199-x. [pdf]
Van Rhijn, J. R., et al. (2018). Foxp2 loss of function increases striatal direct pathway inhibition via increased GABA release. Brain Structure & Function, 223(9), 4211-4226. doi:10.1007/s00429-018-1746-6. [pdf]
Chabout, J., et al. (2016). A Foxp2 mutation implicated in human speech deficits alters the sequencing of ultrasonic vocalizations in adult male mice. Frontiers in Behavioral Neuroscience, 10: 197. doi:10.3389/fnbeh.2016.00197. [pdf]
Share this page